King et al. (2019) accepted at Chemosphere.
https://doi.org/10.1016/j.chemosphere.2019.125126
Authors: Benjamin M King, Nathan J. Janechek, Nathan Bryngelson, Andrea Adamcakova-Dodd, Traci Lersch, Kristin Bunker, Gary Casuccio, Peter S. Thorne, Charles O. Stanier, Jennifer Fiegel
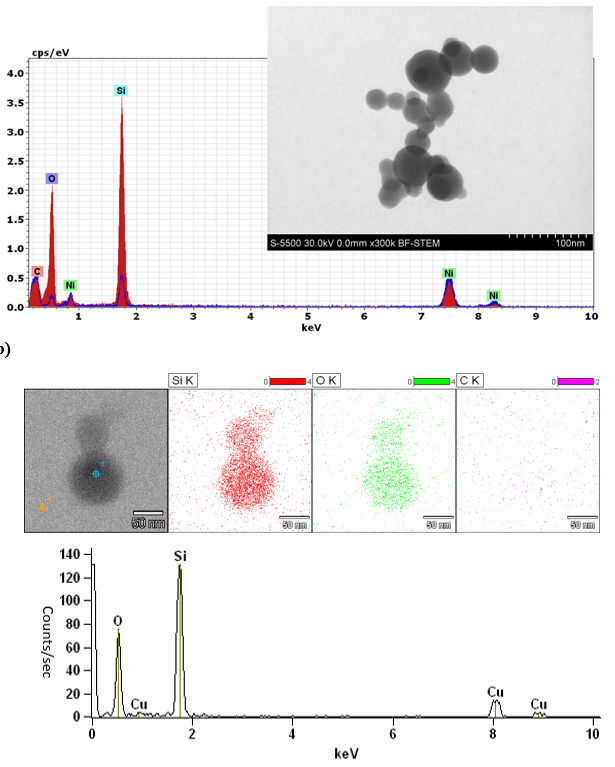
EDS analysis of the chemical composition of particles from (a) TPS sample of Vitrocell exhaust and (b) passive TEM sample of aerosols deposited in Vitrocell wells. (a) EDS analysis of a particle agglomerate (see inset) collected during TPS sampling is shown in red and background grid is shown in blue. (b) Original electron micrograph illustrating the EDS sample region selection (blue crosshair) and comparative background region (orange crosshair) (bar = 50 μm); element mapping of silicon, oxygen, and carbon (bar = 50 μm); and EDS spectra of a particle obtained by passive TEM sampling confirmed that deposited aerosols are derived from siloxanes.
Abstract: To study the fate of cyclic volatile methyl siloxanes (cVMS) undergoing photooxidation in the environment and to assess the acute toxicity of inhaled secondary aerosols from cVMS, we used an oxidative flow reactor (OFR) to produce aerosols from oxidation of decamethylcyclopentasiloxane (D5). The aerosols produced from this process were characterized for size, shape, and chemical composition. We found that the OFR produced aerosols composed of silicon and oxygen, arranged in chain agglomerates, with primary particles of approximately 31 nm in diameter. Lung cells were exposed to the secondary organosilicon aerosols at estimated doses of 54-116 ng/cm2 using a Vitrocell air-liquid interface system, and organic gases and ozone exposure was minimized through a series of denuders. Siloxane aerosols were not found to be highly toxic.
Keywords: oxidative flow reactor, personal care product, photooxidation, Vitrocell, air-liquid interface cell exposure, A549