Chris Brunet's work with CESM2-SHL-Siloxane to probe the impact of temperature dependent oxidation and chlorine oxidation of volatile methyl siloxanes -- is now accepted at ES&T. SHL stands for short-lived halogens. Thanks to Rafa Fernandez and Alfonso Saiz-Lopez for collaborating on the short-lived halogen variant of the model.
URL: https://pubs.acs.org/doi/10.1021/acs.est.5c08897
Plain language summary (with help from AI):
What are VMS?
Volatile methyl siloxanes (VMS) are chemicals widely used in everyday products like shampoos, lotions, and cosmetics. Because they evaporate easily, they can end up in the air and travel long distances.
What was studied?
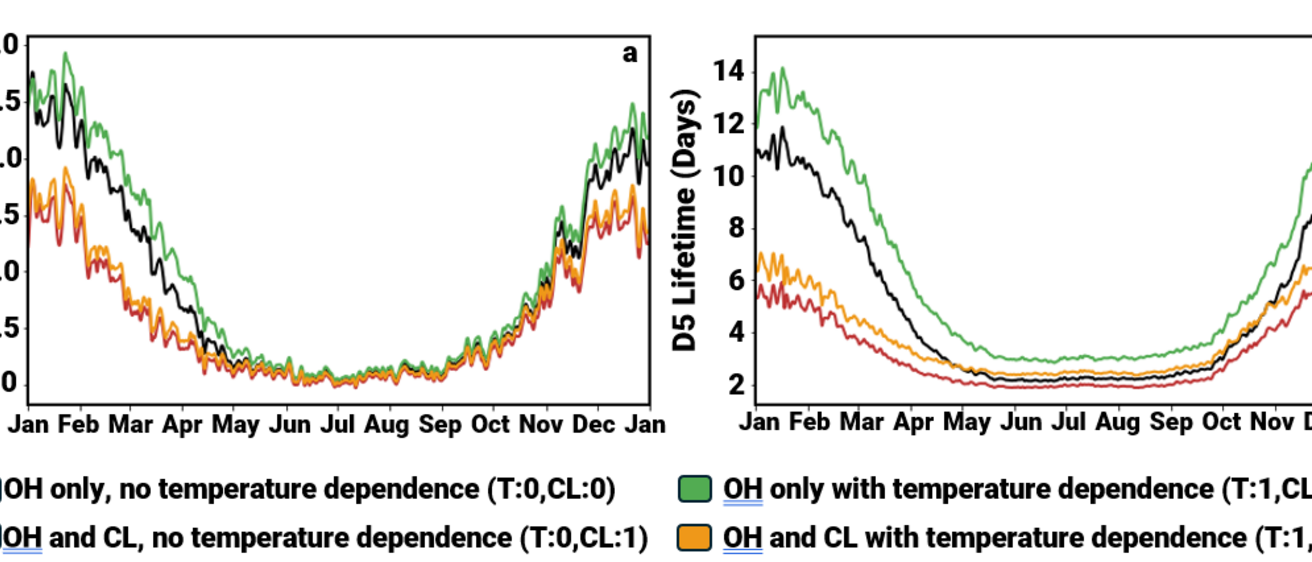
Researchers used a global climate and chemistry model (CESM2-SLH) to better understand how three common VMS compounds (D4, D5, and D6) behave in the atmosphere. They improved the model by adding two important factors:
- Chlorine reactions (never before modeled)
- Temperature effects on chemical reactions (left out of models until now)
Key findings
- Lifetime in air: VMS stay in the atmosphere for about 3–7 days, shorter than earlier studies suggested.
- Concentrations: Levels of D5 were estimated at about 160 ng/m³ in cities and 4.5 ng/m³ in the Arctic.
- Main removal process: Hydroxyl radicals (OH) are still the main way VMS break down, but chlorine reactions also play a big role.
- Transport: Because lifetimes are shorter, VMS might not travel as far globally as once thought.
ABSTRACT: Volatile methyl siloxanes (VMS) are high production volume chemicals found in a wide range of consumer items such as personal care products. VMS have attracted scrutiny due to long-range environmental transport (LRET) concerns. However, their emissions, lifetimes, and concentrations remain uncertain, in part because of limitations of previous atmospheric modeling. Herein, we describe the global modeling of siloxanes: D4 (octamethylcyclotetrasiloxane), D5 (decamethylcyclopentasiloxane) and D6 (dodecamethylcyclohexasiloxane) in the Community Earth System Model (CESM2-SLH) using an updated chemical mechanism that includes chlorine radical oxidation and temperature-dependent reaction rates. With these previously unconsidered factors, we predicted VMS lifetimes ranging between 2.7 and 6.7 days and annual average D5 near-surface concentrations as high as 4.5 ng m-3 in the remote Arctic and 160 ng m-3 in urban areas. These lifetimes and the degree of LRET were significantly lower than previously reported. OH remained the largest loss pathway, although it was decreased at low temperatures relative to previous modeling. The temperature-induced lifetime increase was outweighed by the previously unconsidered chlorine oxidation channel. Model results and previous measurements agreed relatively well but exhibited negative normalized biases (-0.55 to -0.88), particularly in urban areas not well-resolved at global model resolution, and for passive sampler measurements.